- Updated Post-Approval Event Forms
- Tips for Optimal iRIS System Performance
- IRB Limited Meeting Schedule: Impact on Studies Expiring in November, December, January
Updated Post-Approval Event Forms
New versions of the Adverse Event Reporting (AER) Form, Protocol Violation/Incident Report (PV/IR) Form, and Reporting Form have been published. All new versions have an October 2023 date on them.
What are the changes?
- The “revised sites involved” question clarifies what types of study sites should be reported on event forms versus reporting forms (AER and PV/IR forms)
- New follow-up questions for events involving investigational drugs and devices (AER and PV/IR forms)
- New instructions to not attach previously submitted documents or consent forms (AER, PV/IR, and Reporting forms)
Which studies will be impacted?
- NEW Form Submission: If you start a new AER, PV/IR, or Reporting form on or after today, iRIS will automatically present you with the new form version.
- Form Submission in Progress: If you have already started an AER, PV/IR, or Reporting form but have not submitted it yet: Your submission will not be impacted by this update.
- Already Submitted form: If you have already submitted an AER, PV/IR, or Reporting form: Your submission will not be impacted by this update.
Tips for Optimal iRIS System Performance
Here are several practices that all iRIS users should adopt to reduce system slowness and prevent loss of data:
- Click the ‘Save and Continue’ button all the way through the Study Application form after making changes to any section. This will ensure no questions are skipped and prevent possible data losses from changes to show/hide branching.
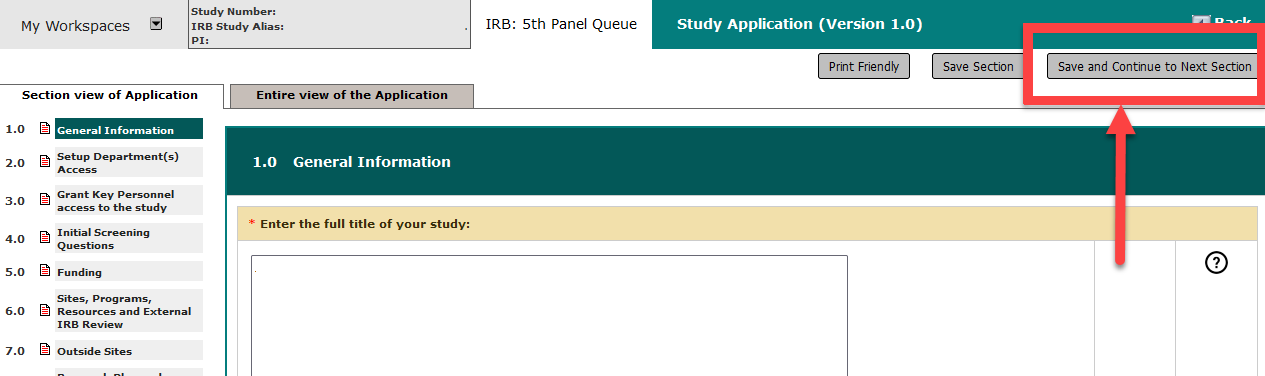
- Do not re-click buttons if the system is responding more slowly than normal. This causes ‘deadlocks’ in the database and makes the system slower for all.
- Be patient and wait for iRIS to complete a task after you click something. Not all buttons have a ‘clock’ icon to show that the system is still working. Look at the browser’s status indicator to determine if the action is still in progress or has been completed. Don’t navigate away from the page or take the next action until after the browser’s status indicator has stopped spinning.
- If iRIS performance is significantly reduced: Log out of the system, close the browser window and all tabs in all sessions and log back into a new browser session or try a different browser. iRIS is load balanced across 4 servers so you will likely not continue to experience performance problems if you start a session on a different server.
IRB Limited Meeting Schedule: Impact on Studies Expiring in November, December, January
Please note that there will be fewer IRB meetings in November, December, and January because of the holiday schedule.
If your study requires review by a convened IRB committee (i.e., “Full Committee”) and is set to expire in November, December, or January, please submit your continuing review at least six weeks prior to study expiration.
Below is a list of common questions:
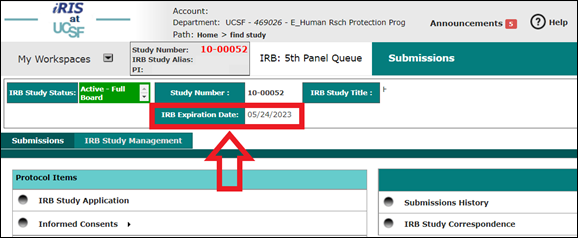
Q) Where can I find my study’s expiration date?
A) If your study has an expiration date, you can find it two different ways:
- Open the study in iRIS and find the expiration date near the top of the screen.
- Check your most recent IRB approval letter. For help finding your approval letters, click on the Tutorial icon in iRIS and then select the “Find My IRB Approval Letters” tutorial from the pop-up window.
Q) How do I know if my Continuing Review submission requires Full Committee review?
A) Your study needs Full Committee review if it is determined to be “greater than minimal risk” by the IRB (check your IRB approval letter for the risk level designation).
Q) My Full Committee study’s expiration date falls within this time frame. What can I do to help prevent it from expiring?
A) Submit it as early as possible (recommended: 6 weeks prior to expiration date) and avoid making modifications to your study materials at the time of Continuing Review, as the review of modifications takes additional time. Whenever possible, get the Continuing Review submission approved first and then submit modifications afterward.
Q) Are there submission deadlines for Continuing Reviews to get on an upcoming meeting agenda?
A) 2023 and 2024 meeting dates and submission deadlines can be found at http://irb.ucsf.edu/irb-rosters-meeting-dates.
Q) How do I know which IRB Committee will review my study?
A) The “Reviewing Committee” is based on your most recent IRB approval letter. Typically, your Continuing Review will go to the same committee that reviewed it previously.
Q) I submitted my Continuing Review. Can I track its review status?
A) Yes. Follow the instructions here: Find My Study
Additional questions? Please contact the IRB using one of the following methods:
- Submit an Ask Andy form (preferred)
- Email [email protected]
- Call 415-476-1814 (Note: you will be prompted to leave a voicemail which will then be forwarded to an IRB Analyst)