- Background
- Definitions
- Guiding Principles
- Exclusion from Study or Use of Surrogate Consent
- Decisional Capacity Assessment for Research and Flowchart
- Methods of Assessment
- Research Outside of California
- Resources
Background
Some studies propose to involve subjects whose capacity to make meaningful decisions is in question because they are "cognitively impaired.“ Such subjects may include mentally disabled persons, and those with diagnosed psychoses, Alzheimer's disease or other cognitive disorders — permanent or temporary. Other subjects may have cognitive impairments as a consequence of severe pain, anxiety or confusion, such as individuals with trauma, cancer or life-threatening illness.
Since many people with these conditions retain the capacity to consent to research, not allowing them to consent for themselves when able could compromise their rights.
Federal regulations, California state code, and UC policy indicate that research studies may involve subjects who have cognitive impairments if adequate safeguards are in place. Individuals signing valid research consent forms for themselves must have adequate decisional capacity, but the relevant regulatory documents do not say how capacity should be determined.
The IRB will consider the following principles:
- Studies should not arbitrarily exclude cognitively impaired subjects if they might be able to give informed consent and there is a chance they could benefit from participation.
- Studies involving subjects with cognitive impairment can only be approved if justified and appropriate additional safeguards are in place. Higher risk studies need a higher level of safeguards.
- The primary additional safeguard for this vulnerable subject population is assessment of decisional capacity.
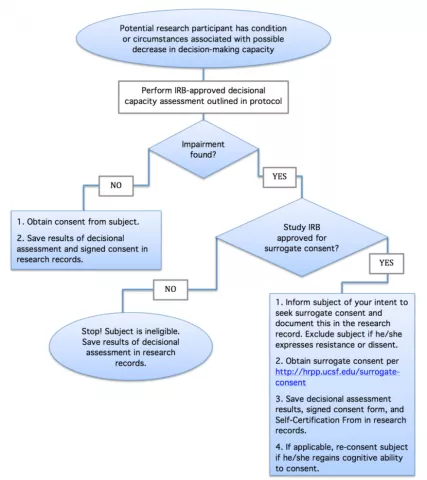
- If adequate decisional capacity is not found upon assessment, the investigator usually needs to either exclude the prospective subject from the study or seek surrogate consent for their participation.
Relevant Regulations and Policies
- Federal regulations (45 CFR 46.111) direct the IRB to approve a study only if it determines that informed consent will be sought from each prospective subject (unless specific exceptions are justified), and that for “subjects…vulnerable to coercion or undue influence, such as …mentally disabled persons…[the study includes] additional safeguards.”
- California law (Health & Safety Code §24178) allows surrogate consent for certain research subjects with cognitive impairment “if (the) person is unable to consent.”
- The University of California provides related UCOP Guidance on Surrogate Consent for Research.
This guidance does not apply to pediatric subjects and emergency research. These guidelines do not supersede more restrictive or specific local requirements or policies.
Definitions
Capacity to Consent (to Research)/Decision Making Capacity: The ability of an individual to understand the choices presented, to appreciate the implications of choosing one alternative or another, and to make and communicate a decision (e.g., whether or not to participate in a study).
Important Notes:
• The capacity to consent is protocol and situation specific. A subject may have the ability to consent to a low-risk protocol in usual circumstances but not have the capacity to consent to a high-risk protocol in a stressful situation.
• “Competence” is a legal term that should not be confused with “decision making capacity.” Someone who was judged legally incompetent to handle their finances may still be able to make a meaningful choice about taking part in a research study. Also, a person who has normal cognitive functioning may be in a circumstance where his/her decision-making capacity to consent is temporarily impaired by physical (e.g., unconsciousness) or emotional trauma (e.g., pain, fear or anxiety)
Surrogate Decision-Maker: A legally authorized representative with reasonable knowledge of a potential research participant who lacks decision-making capacity, as defined under California law (Health & Safety Code 24178).
Guiding Principles
Guiding Principles: The following principles are important to consider when planning research that will or may include those with cognitive impairments:
- Investigators who plan to conduct studies involving subjects with cognitive impairment will need to provide a scientific and/or scholarly justification of the inclusion of this population and assure that appropriate additional safeguards are in place to protect this population from coercion or undue influence.
- Studies should not arbitrarily exclude cognitively impaired subjects if they might be able to give informed, voluntary consent and there is a chance they could benefit from
participation. - Higher risk studies need a higher level of safeguards.
- The primary additional safeguard for this vulnerable subject population is assessment of capacity to consent.
- If adequate decisional capacity is not found upon assessment, the investigator usually needs to either exclude the prospective subject from the study or seek surrogate consent for their participation.
Exclusion from Study or Use of Surrogate Consent
A key choice in study design is whether to 1) exclude people who cannot consent for themselves or 2) include them with surrogate consent from the subject's legally authorized representative.
Note: You must regard a prospective subject's objection or resistance to study participation — in any way or at any time — as a refusal or withdrawal and honor this request immediately, even if a surrogate or the study investigator disagrees with the decision.
Decisional Capacity Assessment for Research
No single set of standards for defining and implementing assessment of decisional capacity has received universal acceptance by experts in the field.
Per UCOP guidance, investigators should "assess subjects on their abilities to understand and to express a reasoned choice concerning the:
- Nature of the research and the information relevant to his/her participation;
- Consequences of participation for the subject’s own situation, especially concerning the subject’s health condition; and
- Consequences of the alternatives to participation. [Applebaum, PS and T. Grisso. "MacCA T-CR: MacAtihur Competence Assessment Tool for Clinical Research. Professional Resource Press, 20QI]"
You should specify who will conduct the decisional capacity assessment, the method(s) of assessment and criteria for identifying incapable subjects. Use the info below to formulate your plan to assess decisional capacity. A less formal procedure to assess potential subjects’ capacity may be permitted if a formal assessment is not feasible or necessary.
Research Outside of California
The determination of capacity to consent depends on the laws and regulations of the local jurisdiction. When investigators plan on enrolling research participants outside California, they should check with UCSF Legal Counsel and/or their collaborators out of state regarding the local consent laws and include Information on the local laws/regulations regarding capacity to consent for research and relevant citations.
Decisional Capacity Assessment Flowchart
Resources
- Guidance and Procedures: Research Involving Persons with Cognitive Impairments (UCLA)
- Decision-Making Capacity Assessment Tool (UCLA)