- Review Process
- Review Outcomes
- Post-Approval Event Review Outcomes
- Conditions of Approval and Approval Documentation
Review Process
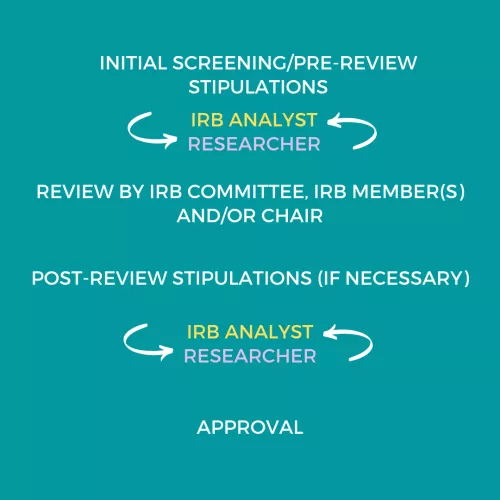
The IRB office utilizes an initial pre-review screening process, during which an IRB analyst reviews each submission for completeness and compliance.
The analyst may ask the PI to make changes to the submission before it is reviewed by the IRB (pre-review). The IRB reviewer(s) may also ask for changes or clarifications, which the IRB analyst will communicate to the research team after IRB review (post-review). See Responding to Stipulations in iRIS for more information.
Notes:
- New studies must meet minimum submission standard requirements before they undergo the pre-review screening process.
- The number of IRB reviewers varies based on the level of review the submission requires. Full committee review studies are reviewed by the IRB committee at a convened meeting, while expedited and exempt studies are reviewed by a small number of IRB reviewers outside of an IRB meeting.
Review Outcomes
One of the following review outcomes is applied to each IRB submission.
Approval Letter Is Issued and Study Can Begin
Study Cannot Begin Until PI Addresses IRB Reviewer Concerns or Required Changes are Addressed by the PI and the Response is Approved by the IRB or the IRB Meets Its Own Criteria for Review
The PI and study contact(s) will receive an email and task in iRIS that identify the review outcome. The Review Response Submission Form in iRIS will list the IRB's requested stipulations or comments (changes or clarifications). Review our tips for responding to post-review stipulations.
Study Cannot Be Approved by the UCSF IRB
Post-Approval Event Review Outcomes
The committee occasionally is asked to make determinations on post-approval events, including internal adverse events or protocol violations and incidents. The committee must determine if the event qualifies as one or more of the following:
- An unanticipated problem involving risk to participants or others
- Noncompliance
- Serious noncompliance
- Continuing noncompliance
More information about these determinations, the review process and reporting/submission guidelines is available on the Adverse Event or Safety Information and Protocol Violation or Incident pages
The IRB may request additional corrective action plans or request that the QIU conduct a directed site visit (currently unavailable). The committee may also suspend or terminate IRB approval, among other actions.